Since most substances in medicines have hygroscopicity, a dry environment is of great importance for the production and storage of medicines. In addition, in the process of pharmaceutical production, the final weight and quality of medicines will be affected by moisture adsorption. Generally, a dry environment can improve productivity and product quality and extend the shelf life of medicines. Generally speaking, the RH should be controlled to 20%-35% and the temperature to 23℃±2 in the process of drug production. Soft capsule production lines are a common production area with dehumidification requirements in pharmaceutical enterprises: the temperature in capsule drying workshops should generally be 23±2 and humidity 20%.
Medicine products, such as powder, tablet, capsule and diagnostic strip, are sensitive ones. Once contacting with the moisture in the air, they tend to lose their effect and shelf life. Such products may stick together, get mildew or crack. Meanwhile, blockage of machinery and pipes may be caused, which may hinder production, transportation and storage. This is a time- and cost-intensive condition that can be avoided. The key is to continuously supervise and monitor air humidity throughout all production links from raw materials to final products to maintain optimum conditions all year round. The pharmaceutical production links in pharmaceutical factories are the guarantee of quality. Therefore, how to strengthen the scientific supervision of drug manufacturing enterprises, standardize various production links and implement quality control throughout the production process plays an important role in improving the quality of medicines and reduce drug accidents. A dehumidifier is special equipment for avoiding the harm of moisture in the process of pharmaceutical production and is used for dehumidifying, removing mildew and improving humid environment. The device is the tool that determines whether the control of production environment in the pharmaceutical factory is effective, while the qualified production environment monitoring is a necessary condition for drug release.
Polder low dew point rotary dehumidifier
The dehumidifier has been widely applied in pharmaceutical factory, pharmaceutical workshop, pharmaceutical warehouse and other places. Dehumidifier adopts the principle of freeze-drying: indrawing moist air, dropping the temperature below the dew point temperature via the evaporator, condensing the moisture in the air into water droplets, and then heating into dry air through the condensing heat by the freeze-compressor for exhaust, so as to achieve the purpose of drying the air.

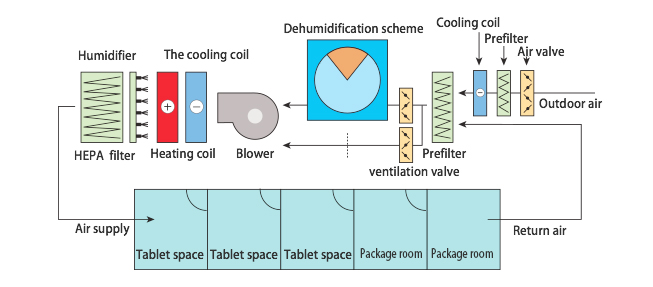
Rotary dehumidifier is usually placed in front of the rear surface cooler to eliminate the moisture, which can avoid the condensation of the rear surface cooler and keep the air duct dry and free from bacteria. The effects of dehumidifier are controlled by the air volume passing through the wheel and the bypass air valve. The more dry air required in the room, the more air passing through the wheel. When the temperature and humidity in the room meet the requirements, more air will pass through the bypass valve and bypass the dehumidification wheel. Such design can keep the indoor RH at ±2%. The typical case application in a pharmaceutical factory is as shown in the following figure: